From the early history of time people were inquisitive about their surrounding, especially the matter. The ancient Greek thinker Damocritus (about 400 BC) began making predictions about the structures of matter. The Roman Lecretius (about 350 years later) took up the same question who predicted that when a piece of gold is cut into several times, a time would come when the piece could not be cut further and they called that invisible small particle of matter as "atom" and following these two ancient philosophers. There appeared a succession of thinkers. European and Arabian whose prediction was end at the beginning of the nineteenth century after around 2000 years in the ideas of John Dalton.
Dalton's Atomic Theory
Dalton introduced his theory as Atomic Theory in 1808 and his theory includes the following points.
1. Mater is made up of small individual particles called atoms.
2. Atoms are indestructible and they cannot be created.
3. The atoms of a particular element are all exactly alike in every way and are different from the atoms of all other elements.
4. Chemical combination takes place between small whole number of atoms.
It seems fairly certain that for most of the nineteenth century, atoms were regarded as very small spherical particles like a every minute lead ball. It was believed atoms are indivisible. This idea was greatly changed in the first half century, mainly by the work of Rutherford.
Atomic Structure:
Less than a century ago, scientist believed that atoms were solid indestructible particles like minute snooker ball, since then they have built up a great deal of evidence concerning the detailed structure of atoms. Experiments involving electrolysis (Faraday's work) suggested that certain compounds contained charged particles called ions; formation of these ions from atoms could be explained only in terms of loss or gain of negative charged particles. This led to the idea that atoms consisted of some other subatomic particles.
Evidence for the existence of subatomic particles:
1897 J.J. Thomson discharge tube experiment with electrons:
In 1897 G.J. Stoney suggested the name electron for the tiny negative particles which made up an electric current. Stoney realized that the experiments involving electrolysis, which Faraday had carried out earlier in the nineteenth century, could be explained in terms of electrons.
However the firm evidence of the existence of electrons was not found until 1897. In that year. J.J. Thomson was investigating the conductivity by gasses at very low pressure.
When Thomson applied 1500 volts across the electrodes of a tube containing a trace of gas, a bright green glow appeared on the glass. The green glow results from the bombardment of the glass by rays traveling in straight line from the cathode when they strike the anode or the glass wall of the tube. Thompson called these rays "cathode rays".
When the rays were deflected by an electric field across a pair of charged plates. The rays moved away from the negative plate attracted towards the positive plate. This suggested that cathode rays were negative. Thompson studied the bending of a thin beam of cathode rays by magnetic and magnetic electric fields and concluded that they consist of electrons tiny negatively charged particles.
By studying the degree of deflection of cathode rays in different magnetic field and electric field. Thompson determined the charge to mass ratio for the electron. He found that the ratio to be e/m = –1.76×108 coulomb/gram
It was further observed by Thompson that the particles present in cathode rays are always same and their e/m value are also the same irrespective of the nature of metal electrode and the nature of the gas used. Therefore it was concluded that cathode rays are up of fundamental particles which were named electrons. Later Robert Milliken, (1909) from his oil drop determined the charge the electron. Which is found to be.
From Thompson e/m Value Millikan's e charge value. The mass of the cathode particle electrons was found to be
This mass is nearly equal to the 1/1837 the mass of one hydrogen atom.
Electron:
Electron is a subatomic particle with unit negative charge and mass equal to 1/1837th of hydrogen atom in amu and its mass is 9.10910-28 gram.
1886 Goldstein discharge tube experiment. Anode rays (positive rays) protons:
Godstein was able to show the presence of positive particles in the discharge tube experiment. The presence of negatively charged particle in an atom suggested that there most be some positively charge particle because the atom on the whole is electrically neutral Goldstein repeated the discharge tube experiment using a perforated cathode tube. It was found that in addition to cathode rays new kinds of rays streaming behind the cathode were found these rays were found to travel in opposite direction to the cathode rays and passed through the holes of cathode. These positively charged particles believed to be formed by collisions between electrons in cathode rays and residual gas. The positive particles move towards cathode where they get electron to form neutral atom or molecules and some of the +ve particle passed through the holes in perforated cathode and straight strike on the glass wall.
 |
Fig: Gold stein discharge tubes
These rays seemed to be emitting from anode and consist of positively charged particle and were named positive rays or anode rays. It was found that the value e/m of particle rays depends upon the nature of the gas taken in the discharge tube.
The lightest positively charged particle in such rays when hydrogen gas is used in the discharge tube Each of the particle has the mass of hydrogen atoms carrying unit positive charge. No positively charged particle of matter, which is smaller than hydrogen yet discovered The masses of other simple ions are found to be approximately integral multiple of masses of these +ve particle of hydrogen which are now called protons. Protons are now considered to be the fundamental particle and a constituent of all atoms. Cont……..

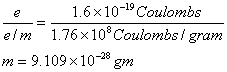




No comments:
Post a Comment